What is the WE-TRUST study
The WE-TRUST Study is a multi-center randomized clinical trial to assess the impact of a ‘Direct to Angio Suite’ (DTAS) workflow on stroke patient outcomes.
Primary objective:
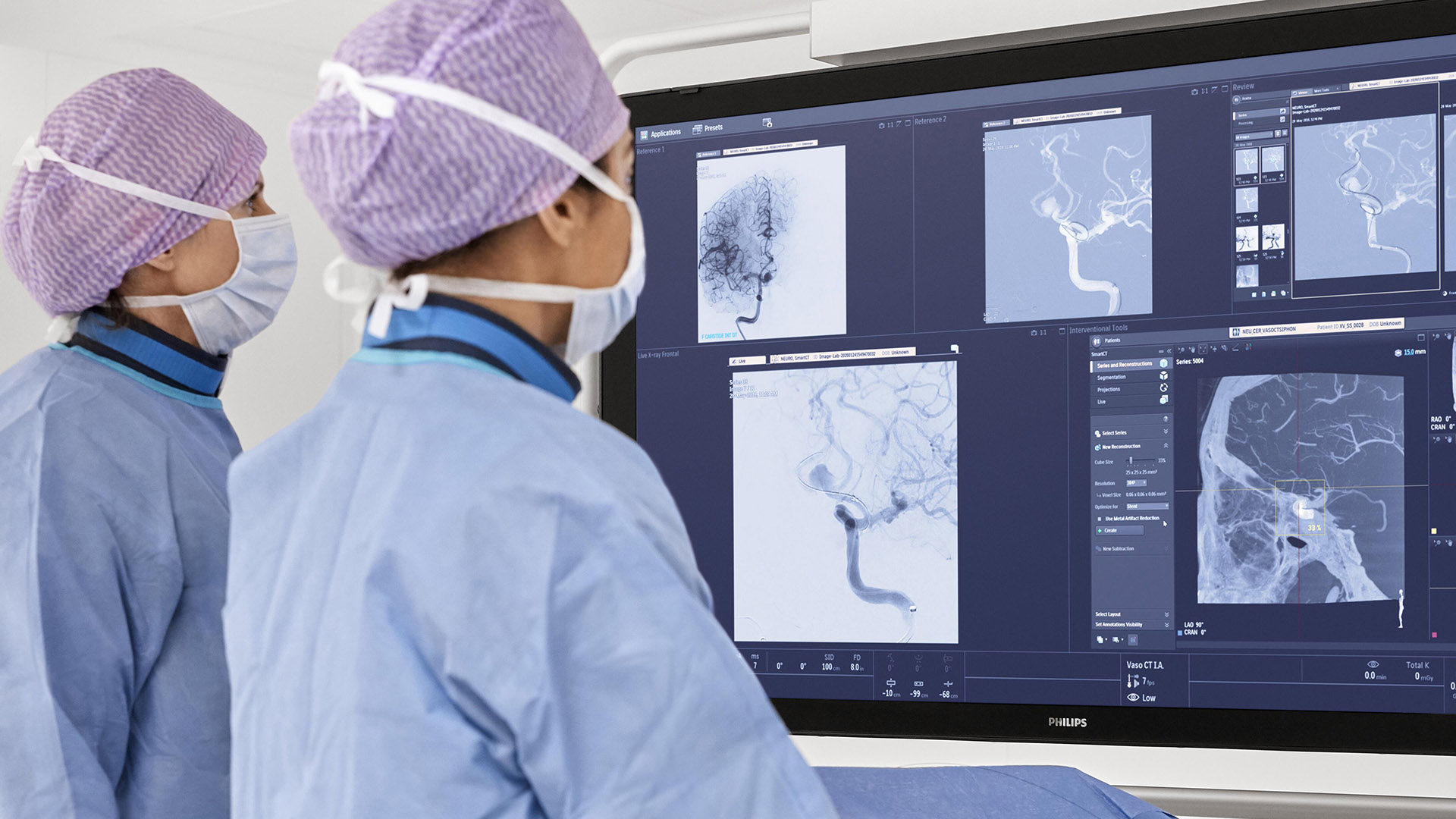
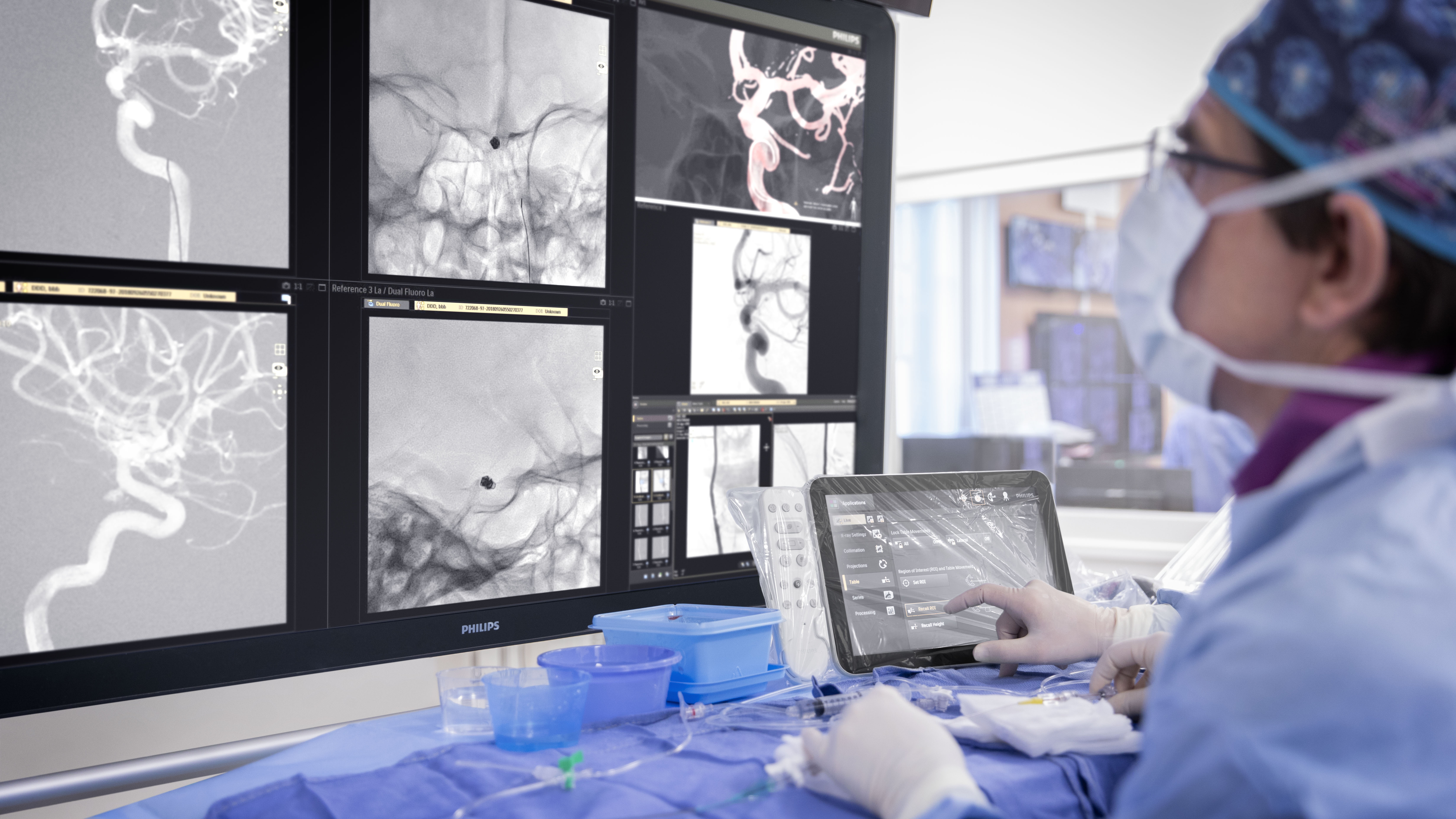
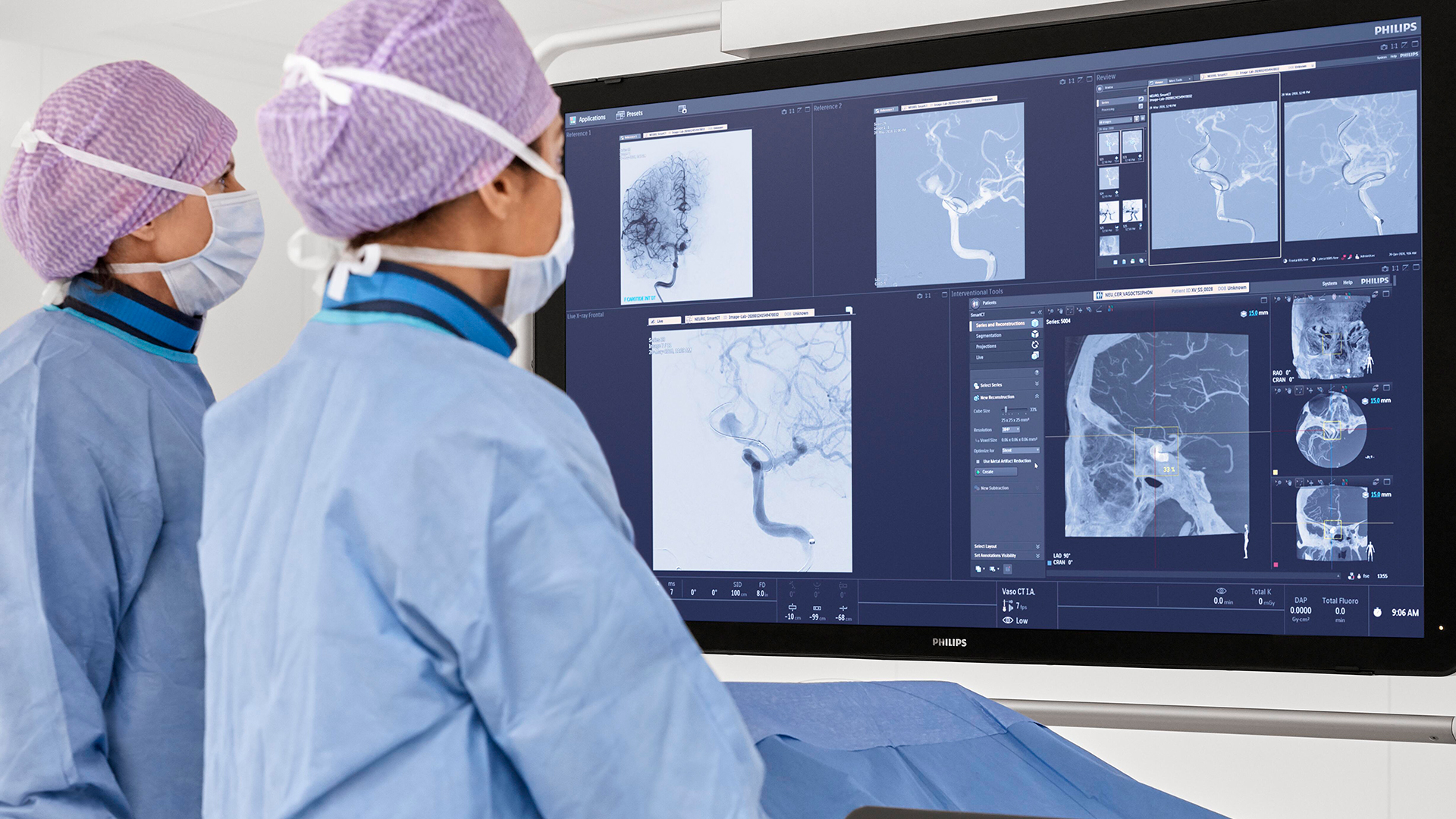
In the DTAS workflow stroke patients are diagnosed and treated in the interventional suite without interruption.Triage imaging is usually performed in the CT/MR suite after which the patient is sent to the interventional suite for treatment. In DTAS approach/workflow the Cone-Beam CT (CBCT) capabilities of the interventional X-ray system are utilized to perform triage, directly followed by stroke treatment. The study will be running in 16 sites to enroll 500+ patients globally.
To demonstrate that the DTAS triage workflow involving CBCT results in superior patient outcome (90 day mRS) in ischemic stroke patients with confirmed Large Vessel Occlusion as compared to the conventional CT/MR triage workflow.
Why was this study performed?
Outcomes for stroke patients are closely tied to how fast they receive treatment: Every 30 minutes delay before treatment reduces the chance of a good outcome by 14%*, and every hour of delay ages the brain by 3.6 years compared to a normally aging brain*.
Currently, when a possible stroke patient arrives at the Emergency Department, typically first a CT or MRI exam is acquired for stroke triage. In case of an ischemic stroke the patient is then treated in an interventional suite. Several studies have indicated that a Direct to Angio Suite (DTAS) workflow can reduce the time to treatment and improve patient outcomes. The WE-TRUST (Workflow optimization to rEduce Time to endovascular ReperfUsion in Stroke Treatment) study aims to provide the most comprehensive assessment to-date that the DTAS triage workflow involving CBCT results in faster treatment and thereby improved patient outcome.
Protocol
High level overview of the protocol
Find study details on clinicaltrials.gov

News
Milestones
FPI (first patient in)
Interim analysis at 75% of patients treated
Publication of study results
Presentation of WE-TRUST study by Dr Nogueira and Dr Ribó at SLICE
First site to start with the study
Interim analysis at 50%
LPI (last patient in)
Milestones
Presentation of WE-TRUST study by Dr Nogueira and Dr Ribó at SLICE
Publication of rationale and design of the study
First site to start with the study
FPI (first patient in)
Interim analysis at 50%
Interim analysis at 75% of patients treated
LPI (last patient in)
Publication of study results
Join the conversation
Join our Linkedin group to be updated on the progress of the study, learnings and examples of implementing the DTAS approach.
Funding & support
Would you like to know more about the Philips vision on the Direct To Angio Suite approach and product solutions? Check the Philips DTAS page.
Philips proudly sponsors and supports this global multi-center study, which is facilitated by current state-of-the-art CBCT capabilities of the Philips’ Neuro Interventional Suite to enable time-efficient triage of acute stroke patient with great confidence.